Ph 5 Diluted With Water
I have made up a 1m citrate buffer at ph 4 5.
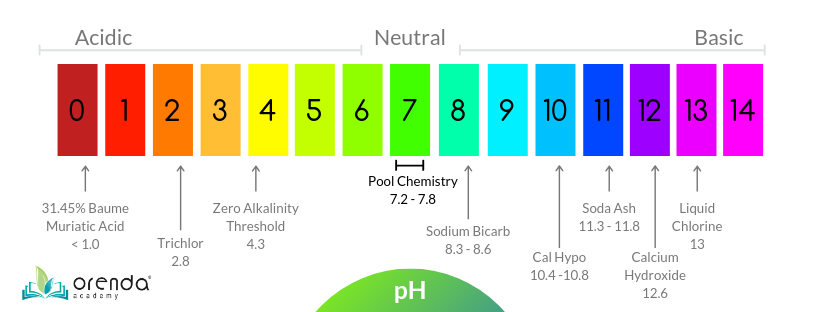
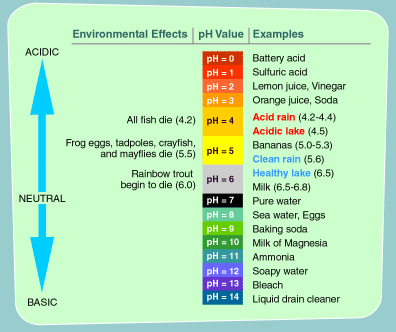
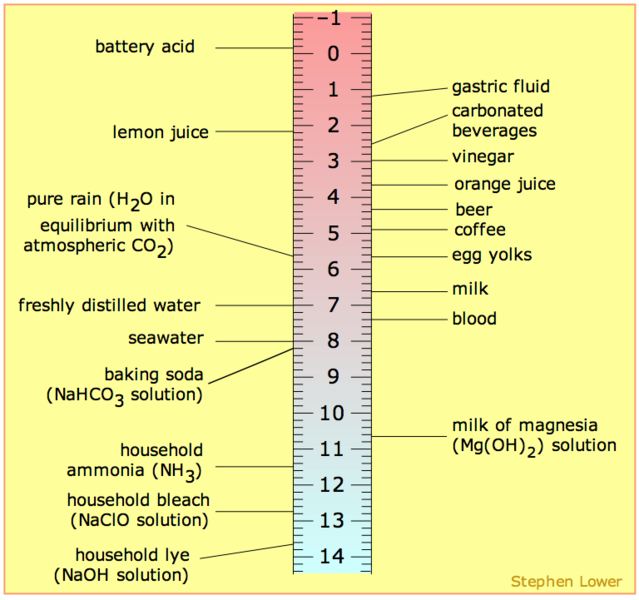
Ph 5 diluted with water. 0 0 1 0. On the ph scale which runs from 0 to 14 the ph level of vinegar is between 2 and 3. Add strong acid to the distiled water. You will see ph of 0 01 m is higher than 0 1 m solution.
The calculated value is a good estimation. When concentrated acid is diluted hydronium ion concentration decreases which results a higher ph value. When an acid like vinegar is diluted with water the concentration of free floating hydrogen ions decreases. Dilution of bases and acids.
So because naoh completely dissociates water the amount of hydroxide ions dissolved in the water is also 2 5 10 3 mol. Never add water to. 0 1 m strong acid is diluted upto 0 01 m. Now let s dilute this stock solution by pouring all 100 ml of our stock solution of naoh aq into a 250 ml volumetric flask and fill that up to the mark with distilled water.
How to dilute strong acid. I have then diluted this solution with deionised water to give concentrations of 50 mm 75 mm and 100 mm and measured their ph. Then calculate ph for two cases and compare two results. Diluting with water will make this solution more neutral meaning it will slowly increase to 7 which is the ph value of pure water used for this dilution.
Please insert three values the fourth will be calculated. You can choose which value you want to leave blank. As a neutral dilution liquid ph value 7 water is estimated. The amount of naoh dissolved in the water hasn t changed that is still 2 50 10 3 mol.